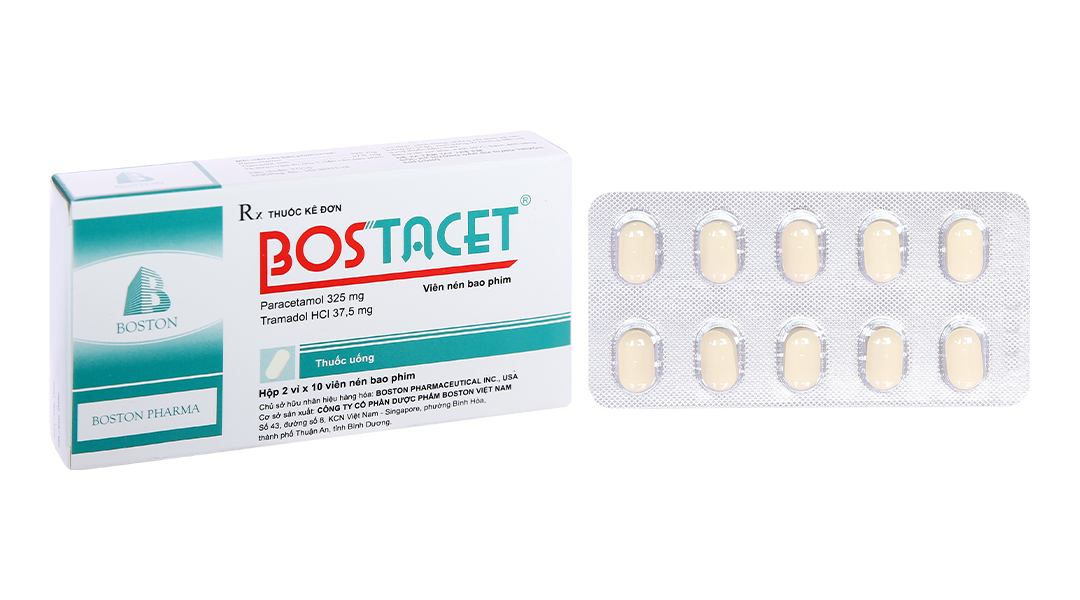
Bostacet 2 boxes = 40 tabs
- Delivered today (order Mon-Fri before 12:00, delivery between 17:00 and 22:00)
- Including shipping costs, sent by blonwe.com
- Pick up at a blonwe.com collection point is possible
- 30 days to change your mind and free returns
- Day and night customer service
Description
PRODUCT INFORMATION
Quick View
1. Ingredients
2. Uses (Indications)
3. How to use – Dosage
4. Contraindications
5. Side effects
6. Notes
7. Pharmacology
8. Additional information
All information below has been compiled by the Pharmacist. However, the content is completely unchanged based on the Instructions for Use, only changing in form.
1. Ingredients
Each film-coated tablet contains:
– Active ingredient:
Paracetamol 325mg.
Tramadol hydrochloride 37.5mg.
– Excipients: Avicel PH 101, pregelatinized starch, sodium starch glycolate, magnesium stearate, silicon dioxide, HPMC, PEG 6000, titanium dioxide, talc, lactose monohydrate, yellow iron oxide.
2. Uses (Indications)
BOSTACET is indicated for the symptomatic treatment of moderate to severe pain.
The use of BOSTACET should be limited to patients with moderate to severe pain and where the combination of paracetamol and tramadol is absolutely necessary.
3. How to use – Dosage
The use of BOSTACET should be limited to patients with moderate to severe pain and where the combination of paracetamol and tramadol is absolutely necessary.
Adjust the dose for each patient according to the intensity of pain and the patient’s response. The lowest effective dose should be used for pain relief. Do not use more than 8 tablets/day (equivalent to 300mg tramadol hydrochloride and 2600mg paracetamol). The interval between doses should not be less than 6 hours.
Adults and children 12 years of age and older
The starting dose is 2 tablets, with additional doses as needed, but not more than 8 tablets per day. The interval between doses should not be less than 6 hours.
In no case should BOSTACET be used for longer than necessary. If repeated use or prolonged treatment with BOSTACET is necessary because of the nature and severity of the disease, caution and regular monitoring (including periods of discontinuation if possible) should be used to assess the need for continued treatment.
Children under 12 years of age
The drug is not recommended for children under 12 years of age due to concerns about safety and efficacy.
Elderly
No dosage adjustment is usually required in patients over 75 years of age who do not have clinically evident hepatic or renal impairment. However, it should be noted that the elimination half-life is prolonged in patients over 75 years of age. Therefore, depending on each patient, the interval between doses may be extended if necessary.
Renal impairment/ Haemodialysis
Because of the presence of tramadol, tramadol hydrochloride/paracetamol is not recommended in patients with severe renal impairment (creatinine clearance < 10 mL/min). In case of moderate renal impairment (creatinine clearance from 10 to 30 mL/min), the interval between doses should be increased to approximately 12 hours. Since tramadol is only very slowly eliminated by haemodialysis or haemofiltration, it is not necessary to supplement the dose of analgesic after dialysis.
Hepatic impairment
The elimination time of tramadol is increased in patients with impaired liver function. Therefore, depending on each patient, the dose interval should be considered when using the drug for this patient group.
Because the product contains paracetamol, BOSTACET should not be used in patients with severe hepatic impairment.
How to use
– BOSTACET film-coated tablets are taken orally with water, swallowed whole, do not break or chew the tablets.









Reviews
There are no reviews yet.